January is National Blood Donor Month. President Lyndon B. Johnson signed a proclamation designating January as National Blood Donor Month (NBDM) on December 31, 1969 (AABB, 2021). Originally meant to honor blood donors and to encourage more people to give blood during the winter months when blood supplies are traditionally low due to lagging donations from the holidays and cold and flu season, it is not uncommon for there to be shortages in the blood supply during January.
January 2022 is no exception! According to a joint statement put out by the Association for the Advancement of Blood and Biotherapies (AABB), America’s Blood Centers, and the American Red Cross, the nation’s blood supply is currently at a critically low level. The joint statement explains that blood centers have reported less than a one-day supply of certain blood types. As of yesterday, January 11, 2022, 36% of the country’s blood centers have a one-day supply or less, and only 2% of the nation’s blood centers reported having a three day or more supply (enough to meet normal operating demands) according to America’s Blood Centers. The American Red Cross, which supplies 40% of the nation's blood supply, states that the organization is facing “its worst blood shortage in over a decade, posing a concerning risk to patient care” (American Red Cross, 2022).
While the blood supply is traditionally lower in January, the current COVID-19 case surge, winter storms, blood drive cancellations, staffing challenges and donor eligibility misinformation have all posed additional threats to the current blood supply. The American Red Cross states that an overall 10% decline in donations since March 2020, and a 62% decline in college and high school blood drives resulting from the pandemic have also influenced the blood shortage crisis (American Red Cross, 2022). Meanwhile, the demand for blood has not decreased. According to the joint statement, “blood donations are needed now to avert the need to postpone potential lifesaving treatments” (AABB, America’s Blood Centers, American Red Cross, 2022).
Regardless of your blood type, donating can make a positive impact! The requirements for donating blood are simple: Donors must be healthy and feeling well, be at least 17 years old, and weigh 110 pounds or more. If you have donated blood recently, wait at least 56 days before making another donation.
You can participate in National Blood Donor Month and help replenish critically low blood supplies by scheduling an appointment to donate blood today! The American Red Cross is currently offering extra incentives to blood donors who donate by January 31, 2022 including a chance to win a trip to Super Bowl LVI! Contact one of the following organizations to find a blood collection site near you:
- AABB: www.aabb.org; 301-907-6977
- America’s Blood Centers: www.americasblood.org; 202-393-5725
- American Red Cross: www.RedCrossBlood.org; 800-RED-CROSS (800-733-2767)
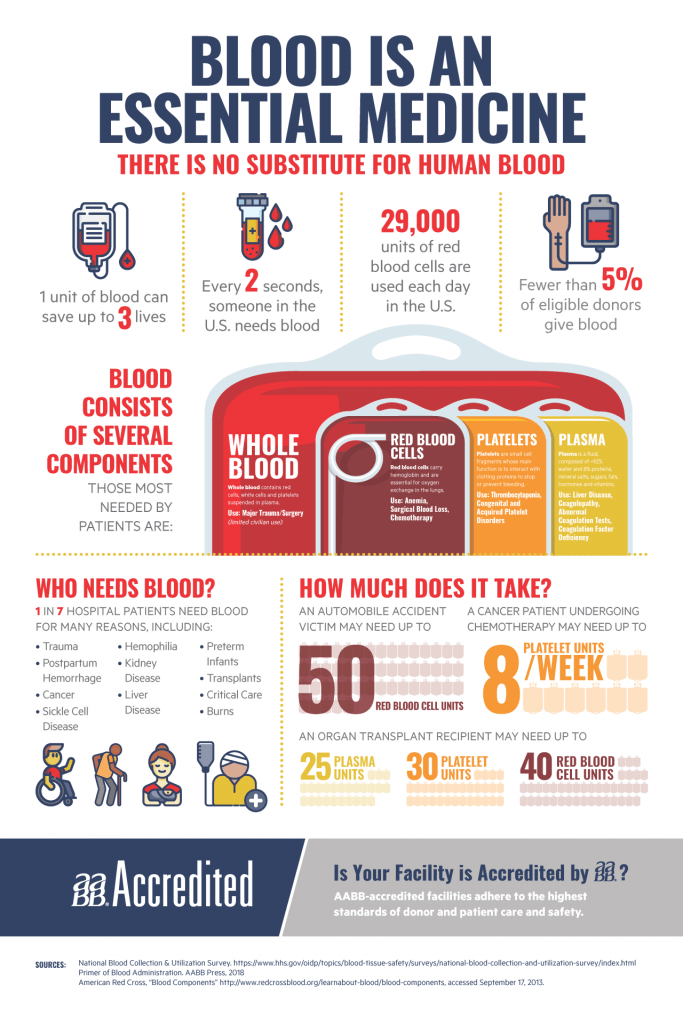
Donating blood is safe as blood donation sites have adapted safety protocols to comply with local, state, and federal safety regulations to protect blood donors and staff. Donors and staff are required to wear masks, donor beds meet social distancing needs, and cleaning processes have been enhanced. One unit of blood can save up to three lives, yet less than 5% of eligible donors give blood. Donating blood is an easy and free way you can make a positive contribution during these times, so celebrate National Blood Donor Month by donating in January!
References
America’s Blood Centers’ (January 11, 2022). America’s blood centers’ website. https://americasblood.org/
American Red Cross (2022). Worst blood shortage in over a decade. National blood crisis. https://www.redcrossblood.org/donate-blood/dlp/red-cross-national-blood-shortage-crisis.html
Association for the Advancement of Blood & Biotherapies (AABB). (2021). National blood donor month. https://www.aabb.org/for-donors-patients/national-blood-donor-month
Association for the Advancement of Blood & Biotherapies, America's Blood Centers, American Red Cross (January 10, 2022). Joint statement: Blood donors urgently needed during national blood donor month and throughout the year. National blood donors month. https://www.aabb.org/docs/default-source/default-document-library/positions/joint-statement-blood-donors-urgently-needed-during-national-blood-donor-month.pdf?sfvrsn=4506f62e_6