 To complement our research of the optical band gap in flames, our group has undertaken a series of computational chemistry studies. We have examined the relationship of the band gap to the molecular structure of PAH molecules, looking at size, topology, and the formation of agglomerates. Our most recent investigations have included the examination of oxygen-containing PAH.
To complement our research of the optical band gap in flames, our group has undertaken a series of computational chemistry studies. We have examined the relationship of the band gap to the molecular structure of PAH molecules, looking at size, topology, and the formation of agglomerates. Our most recent investigations have included the examination of oxygen-containing PAH.
We have performed our computational studies using primarily NWChem and GAMESS-US.
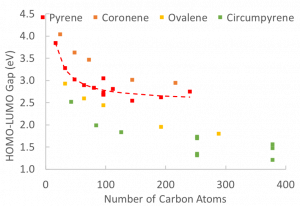
In general, as a PAH increases in size its band gap decreases. However, the particular topology of the PAH molecule also has a role in the size of the band gap. If a PAH molecule stacks with a similarly sized molecule, the band gap will decrease, however if disparate PAH molecules stack, the band gap of the particle is dominated by the molecule with the lowest band gap.
