Evaluating Foam Degradation and Fuel Transport Rates through Novel Surfactant Firefighting Foams for the Purpose of AFFF Perfluorocarbon Replacement
K. Hinnant et al., Eastern States Meeting of the Combustion Institute, March 2018
Abstract: Perfluorocarbon surfactants are used in aqueous film forming foams (AFFF) world-wide to suppress Class B pool fires. We believe the rapid fire suppression capabilities of perfluorocarbon surfactants are related to their ability to generate stable foams and foams that are resistive to fuel transport. Through a research program aimed at replacing perfluorocarbon surfactants in AFFF, we have evaluated foam degradation and fuel transport through foams at elevated temperatures for various classes of surfactants. The surfactants were evaluated individually and in a mixture with a hydrocarbon surfactant (Glucopon) to emulate a previously designed reference AFFF. This data was compared to the performance of a perfluorocarbon surfactant reference AFFF and a commercial AFFF.
Fast fire suppression is dependent on a foams ability to block fuel vapors traveling between the burning fuel pool below and the flame above, and maintain physical coverage over the fuel pool. Foam degradation is effected by heat from the fuel pool, heat from the fire above, and physicochemical interactions between the foam and fuel. Fuel transport through the foam is influenced by diffusion properties between surfactants in the foam and fuel as well as the foam layer thickness which is significantly impacted by increased foam degradation. By measuring foam degradation and fuel transport through the foam at elevated temperatures, we are better able to understand how fuel transport changes with increased foam degradation which mimics some characteristics of a flame environment.
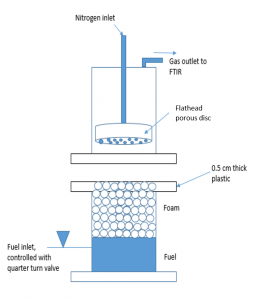
Foam solutions were made by measuring the critical micelle concentration for individual surfactants and surfactants mixed in a 3:2 volumetric mixture with the surfactant and Glucopon, respectively. The foam solutions had surfactant concentrations 6 times greater than the critical micelle concentration. Foams were then generated from the foam solutions to measure foam degradation and fuel transport through the foam. Foam degradation was measured by placing 4 cm of foam above a heated n-heptane pool and monitoring the change in foam height over time. Fuel transport through the foam was measured by placing 4 cm of foam above a heated fuel pool in a specially designed fuel flux apparatus that monitored the concentration of fuel vapors above the foam over time using a nitrogen sparger and an FTIR. Surfactants analyzed included hydrocarbon surfactants, silicone surfactants, and sulfonated surfactants. From the currently evaluated surfactants, none have matched the foam degradation or fuel transport performance of the reference or commercial AFFF. However, certain surfactants performed better than others indicating potential directions for future AFFF surfactant replacement.
Presentation available here: Eastern States CI Presentation 3_2_18-250wawi