May 31, 2017
In 2016, an interesting and potentially practice-changing articles was published: the PESIT trial by Prandoni et al.. The prospective study utilized 11 Italian hospitals and evaluated all patients for pulmonary embolism (PE) who were admitted to the hospital for first-time syncope. The results made for headlines; pulmonary emboli were found in 1 in 6 patients, and was broadcast by major news outlets including NPR and Medscape. With the headlines came a debate amongst physicians about just how useful--if not harmful this study might be.
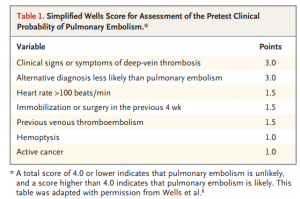
Prior to the PESIT trial, there was scant data on the prevalence of PE in patients admitted for syncope. The authors created a multicenter, cross-sectional study with 11 hospitals in Italy to evaluate the frequency of PEs in patients admitted for syncope. Each patient was risk stratified with a Simplified Wells Score (see Table 1) Those with a score greater than 4 or with a positive D-dimer underwent CT angiography or V/Q scan to evaluate for pulmonary emboli.
Of 2,584 patients who were evaluated by the Emergency Department for syncope, 560 patients were included in the study. Of these, 230 patients received CTA or V/Q scans, and 97 patients were found to have PEs.
The Objective Results:
- 2584 patients assessed
- 560 included (Mean age = 76 )
- 1867 patients discharged and therefore excluded
- 157 inpatients further excluded
- Prior episodes of syncope, anticoagulated, pregnant excluded
- 97/560 (17.3%) were found to have PE
- 230/560 were high risk -> CTA or V/Q scan
- 180 had CTA, 1 had autopsy
- 73/180 had PE
- 49/73 (67%) had a main pulmonary artery or lobar artery PE
- 19/73 (26%) had segmental PE
- 5/73 (7%) had subsegmental PE
- 73/180 had PE
- 49 had V/Q
- 330/560 were low risk -> no imaging
The result of this study carries a bold statement. 1 in 6 of patients hospitalized for syncope have a PE, therefore perhaps we should consider testing syncope patients for PE even when we think the symptoms are caused by something else given the high prevalence of disease. However, without reading the paper in detail may result in lowering the threshold to test for PE and potentially an overutilization of D-dimer, CTA, and/or V/Q scans. Below,we present the pros and cons of this paper to better inform whether to consider PE in your next syncope patient.
Practice Changing:
Bottom Line: The PESIT trial is a well designed study that should increase our consideration of PE during syncope workups, especially in the geriatric population. When a pulmonary embolism causes syncope, a missed diagnosis may cause significant complications and patient safety issues.
Although we are getting better at catching PEs, they can often be subtle and difficult to diagnose. If a casual reader were to browse through the abstract or to base their judgement on a tertiary news source, they would miss the true value behind this study. It actually calls for a closer examination of PE in older patients that are admitted for first-time syncope, not for testing everyone. Those who are low risk and considered safe for discharge were not included in this study, and this study should not impact our practice in these patients. Those at high enough risk to warrant admission may need a second look. In those with prior PEs, undetermined cause of syncope, or clinical features of PE (respiratory distress, tachycardia, hypotension, or signs of DVT) we should have an increased suspicion of pulmonary embolism.
This conclusion results from the question of, how exactly do we translate an inpatient study and apply it to the ED setting? What population of syncope seen in the ED do we even consider PE in? Based on the population you see and ED you work in, admission criteria for syncope is likely to differ. For appropriate reasons, there wasn’t a standard for syncope admission in this study. However, if we refer to Table 1 of the article, a useful data point is the age of the study population. Not so surprisingly, the mean and median age of the study population was 76 and 80, respectively. It makes sense. Geriatric patients tend to have higher admission rates since they have more comorbidities, less reserve, and a larger number of possible etiologies for their symptoms.
Although there is a different discussion to be had about the causality, it is still standard of care to treat PE even if it exists as a non-etiologic diagnosis for their syncope admission. 67% of patients with a CT positive for PE had a significant embolus that could be related to the syncope they experienced. Only 7% of CT-PE positive patients had subsegmental PEs. The best we can take from this data is to consider PE as an association in the geriatric population that presents as first-time syncope.
Not so fast:
Bottom Line: This inpatient study has the dangerous implication of resource overutilization and the results are difficult to translate to other countries and the ED.
There are radiation and potential contrast risks, workflow, and healthcare costs to consider especially if patients or providers do not understand the implications behind this study. The study does not imply that 1 in 6 patients syncopized because of a PE. It just reports that of the patients admitted for syncope, 1 in 6 were found to have a PE. Association, but not causality is what this is. 12.7% of those found to have pulmonary emboli had already been given an alternative diagnosis. Many of these patients may have presented to the ED with an emboli, and others may have been asymptomatic as the clinical significance of these findings is not known.
While the authors focus on the number of patients admitted, they do not account for the 2584 patients screened in the ED. Additionally, many of the patients found to have PEs showed clear evidence of DVT and would have a high Well’s score, which should be identified in the ED prior to admission. When all 2584 patients are taken into consideration, you see a much more typical rate of PEs that many of us are more used to seeing, near 5-10%. It is difficult to translate the algorithm and results of the study across the world from Italy to the US. Do we also discharge 72% of our first time syncope patients? Do we D-dimer all of our syncope patients? The clear answer is no. Despite the authors’ claim that the “standardized protocol” for the diagnostic workup of syncope was based on international guidelines - there is no mention in either guideline about the use of D-dimer or even Well’s in the initial consideration for syncope (1,2).
Summary:
Bottom Line: This study is not practice-changing, but it should increase our awareness for the prevalence of PE in the geriatric population that presents with first-time syncope. If you decide to change your practice, consider the downstream consequences.
There may be some benefit in considering PE in the differential in the admitted geriatric patient with first-time syncope. However, the guidelines (1,2) from both cardiology societies in the United States and Europe do not even discuss the use of D-dimer or Well’s criteria in the initial workup for syncope. Although these tools are an acceptable method for ruling out PE, PE may not even be on the differential for a first-time syncope patient.
This study potentially opens a can of worms. If we start to screen the geriatric population more for PEs, what are the downstream consequences? We will begin to adopt age-adjusted D-dimers more? Will we seen an increase in V/Q scans in patients with kidney disease? Will we see an increase in kidney disease from the contrast used in CTAs? What is the appropriate management for the subsegmental PEs that we find? Will we see an increase in bleeding complications such as GI bleeds or head bleeds in the fall-prone geriatric population? What implications will this have for ER workflow? This article, like many others, is merely a beginning step towards more inquiry. It is not practice-changing, but should increase awareness for the prevalence of PE especially in our geriatric population.
What this paper does do is to open an avenue to have discussions with patients. In the world of the internet and immediate access to information, patients come with new expectations. While we may see an increase in patients or other physicians requesting CT scans and D dimer testing, we have the opportunity to better inform our patients. Identifying your patient’s concerns and addressing them with evidence-based medicine should be the foundation of our practice. Being able to translate the evaluation for DVT and PE for a lay person can be difficult, but may produce a more informed and empowered patient. This paper, and the media attention it received, is a launch point for a larger discussion we should all be having with our patients.
- Task Force for the Diagnosis and Management of Syncope, European Society of Cardiology, European Heart Rhythm Association, Heart Failure Association, Heart Rhythm Society. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009;30:2631-2671
- Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation 2006;113:316-327
Will Denq, MD & Evan Kuhl, MD are both Emergency Medicine Residents at The George Washington University Hospital