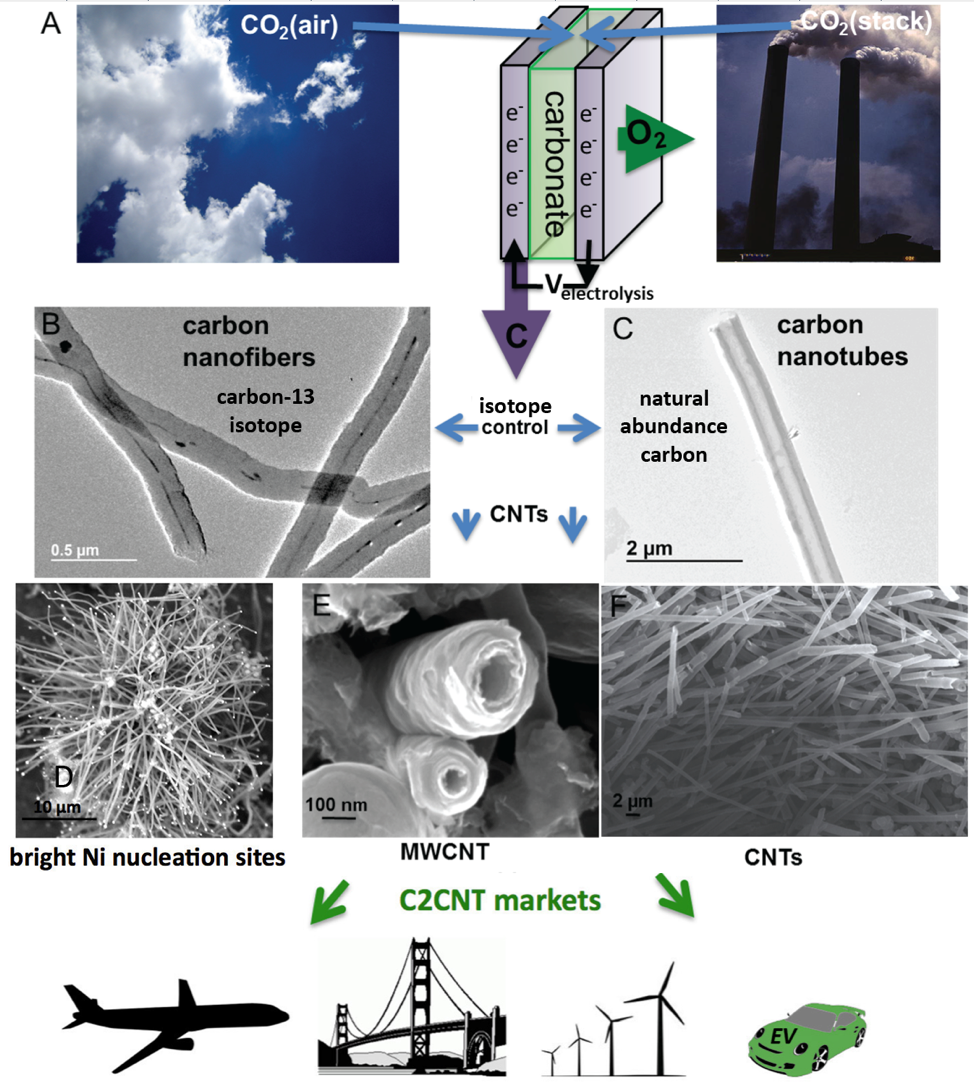
We’ve introduced a new chemistry to transform CO2 into a portfolio of useful, uniform carbon nanomaterials, such as carbon nanotubes, graphene, carbon nano-onions, carbon nanoplatelets, carbon nano-scaffolds, as a facile carbon capture process. For splitting CO2 to carbon nanotubes (and oxygen) the process is termed “C2CNT” (CO2 to Carbon Nanotubes). C2CNT is the direct high yield, low energy, inexpensive transformation of carbon dioxide from the atmosphere, smokestacks, or other anthropogenic sources into valuable, useful, compact, stable products. Since the industrial age, massive quantities of greenhouse gases, principally carbon dioxide, have been released into our atmosphere. With C2CNT, CO2 is bubbled into carbonate and electrolyzed, evolving oxygen at the anode and producing carbon nanotubes (CNTs) grown at the cathode. CNTs, with remarkable properties of lightweight strength greater than steel, flexibility, and high conductivity, provide the most compact form to capture CO2 and mitigate climate change. The C2CNT market can provide lightweight, cheap replacements for metals, new bullet and “taser” proof textiles, stronger cement composite building materials, and expanding applications in industrial catalysis, batteries and nanoelectronics.”

- Wang, Licht, Liu, Licht, “One pot facile transformation of CO₂ to an unusual 3‑D nano‑scaffold morphology of carbon” Scientific Reports, 10, 21518 (2021); Click here for open access article
- Wang, Licht, Liu, Licht, “Calcium metaborate induced thin walled carbon nanotube syntheses from CO₂ by molten carbonate electrolysis” Scientific Reports, 10, 15146 (2020); Click here for open access article
- Ren, Yu, Licht, Peng, Lefler, Liu, Licht, “Recent Advances in Solar Thermal Electrochemical Process (STEP) for Carbon Neutral Products and High Value Nanocarbons,” ACCOUNTS of Chemical Research, 52, 3177 (2019); Click here for article
- Liu, Wang, Licht, Licht, “Transformation of the greenhouse gas carbon dioxide to graphene,” J. CO₂ Utilization, 36, 288 (2020); Click here for articleOpens in a new window
- Wang, Sharif, Liu, Licht, Lefler, Liu, Licht, “Magnetic carbon nanotubes: Carbide nucleated electrochemical growth of ferromagnetic CNTs from CO₂,” J. CO₂ Utilization, 40, 101218 (2020); Click Here for Article
- Liu, Ren, Licht, Wang, Licht, “Carbon Nano-Onions Made Directly from CO₂ by Molten Electrolysis for Greenhouse Gas Mitigation", Advanced Sustainable Systems, 52, DOI: 10.1002/adsu.201900056, 190056 (2019); Click here for article
- Licht, Liu, Licht, Wang, Swesi, Chan, “Amplified CO₂ reduction of greenhouse gas emissions with C2CNT carbon nanotube composites,” Materials Today Sustainability, 6, 100023 (2019); Click here for article
- Johnson, Ren, Lefler, Licht, Vicini, Liu, Licht, “Carbon nanotube wools made directly from CO2 by molten electrolysis: Value Driven pathways to carbon dioxide greenhouse gas mitigation,” Materials Today Energy, 5, 230 (2017); Click here for article
- Ren, Licht, “Tracking airborne CO2 mitigation and low cost transformation into valuable carbon nanotubes,” Scientific Reports - Nature.com, 6, 27760 (2016). Click here to access article
- Ren, Johnson, Singhal, Licht, “Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes,” J. CO2 Utilization, 18, 335 (2017).
- Licht, “Co-production of cement and carbon nanotubes with a carbon negative footprint,” J. CO2 Utilization, 18, 378 (2017).
- Lau, Dey, Licht, “Thermodynamic assessment of CO2 to carbon nanofiber transformation for carbon sequestration in a combined cycle gas or a coal power plant," Energy Conversion & Management, 122, 400 (2016). Click here to access article
- Wu, Li, Ji, Liu, Li, Yuan, Zhang, Ren, Lefler, Wang, Licht, “One-Pot Synthesis of Nanostructured Carbon Material from Carbon Dioxide via Electrolysis in Molten Carbonate Salts,” Carbon, 106, 208 (2016). Click here to access article
- Licht, Douglas, Ren, Carter, Lefler, Pint, "Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes," ACS Central Science, 2, 162 (2016). Click here to access article
- Dey, Ren, El-Ghazawi, Licht, "How does amalgamated Ni cathode affect Carbon Nanotube growth? A density functional theory study," RCS Advances, 6, 27191 (2016).
- Ren, Li, Lau, Li, Gonzalez-Urbina, Licht "One-pot synthesis of carbon nanofibers from CO2," Nano Letters, 15, 6142 (2015). Click here to access article
- Ren, Lau, Lefler, Licht, "The minimum electrolytic energy needed to convert CO2 ...," J. Phys. Chem. C, 119, 23342 (2015). Click here to access article
- Licht, "High solar efficiency utilization of CO2" in Green Carbon Dioxide, Wiley (2014).
- Licht, Cui, Wang, "STEP Carbon Capture: the barium advantage," J. CO2 Utilization, 2, 58 (2013).
- Licht, Wang, Wu, "STEP-A solar chemical process to end anthropogenic global warming II: experimental results," J. Phys. Chem. C, 115, 11803 (2011).
- Licht, Wang, Ghosh, Ayub, Jiang, Ganley, "A New Solar Carbon Capture Process: Solar Thermal Electrochemical Photo (STEP) Carbon Capture," J. Phys. Chem. Lett., 1, 2363 (2010).
- Licht, “STEP: A solar chemical process to end anthropogenic global warming,” J. Phys. Chem. C, 113, 16283-16292 (2009).
Licht Group Solar Energy Research
 A new solar process has been introduced, the STEP process, which can efficiently remove carbon from the atmosphere and generates the staples needed by society, ranging from fuels, to metals, bleach and construction materials, at high solar efficiency and without carbon dioxide generation. STEP is a path to lower carbon dioxide in the atmosphere to pre-industrial concentration levels.
A new solar process has been introduced, the STEP process, which can efficiently remove carbon from the atmosphere and generates the staples needed by society, ranging from fuels, to metals, bleach and construction materials, at high solar efficiency and without carbon dioxide generation. STEP is a path to lower carbon dioxide in the atmosphere to pre-industrial concentration levels.
By using the full spectrum of sunlight, STEP captures more solar energy than the most efficient solar cell. STEP's use of concentrated sunlight and concentrated reactants drives high, industrial rate, of productions in new high temperature molten salt synthesis cells. Photoelectrochemistry is the study of light driven electrochemical processes. Highlights of the Licht Group photoelectrochemical studies include:
- The STEP process decreases atmospheric carbon dioxide: 2009 to present (see below), such as in: Recent Advances in Solar Thermal Electrochemical Process (STEP) for Carbon Neutral Products and High Value Nanocarbons,” ACCOUNTS of Chemical Research, 52, 3177 (2019); Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes,” J. CO2 Utilization, 18, 335 (2017). Tracking airborne CO2 mitigation and low cost transformation into valuable carbon nanotubes,” Scientific Reports – Nature.com, 6, 27760 (2016) and in “One-pot synthesis of carbon nanofibers from CO2,” Nano Letters, 15, Aug. 3 (2015).
- >> 100 photoelectrochemical studies, chapters & books are included in the Licht group select publications.
- Demonstration of the dominance of solution speciation effects on photoelectrochemical charge transfer including the cesium enhancement of solar cell photovoltages, such as: “A Description of Energy Conversion in Photoelectrochemical Solar Cells,” Nature, 330, 148 (cover article, 1987) and “Efficient photoelectrochemical solar cells,” Nature, 345, 330 (1990).
- High efficiency solar cells that works in the dark (illuminated semiconductor driven in-situ electrochemical charge storage), such as “A Light Variation Insensitive High Efficiency Solar Cell,” Nature, 326, 863 (1987) and “Light Invariant, Efficient, … AlGaAs/Si/Metal Hydride Solar Cell” Applied Physics Letters, 74, 4055 (1999).
- The theoretical & experimental development of multiple band-gap photoelectrochemistry, such as ” Multiple Bandgap Semiconductor/Electrolyte Solar Energy Conversion ” Journal of Physical Chemistry, B, Feature Article, 105, 6281 (2001).
- Demonstration of fullerene photoelectrochemical solar cells, such as “Electrochemical Potential Tuned Solar Water Splitting” Chemical Communications, 3006 (2003).
Demonstration of highest solar efficiency of water splitting to generate hydrogen fuel, such as "Electrochemical Potential Tuned Solar Water Splitting" Chemical Communications, 3006 (2003).
 |
 |
 |
 |
The STEP portfolio of processes to eliminate carbon dioxide emission is growing rapidly and includes:
STEP fuel (CO2 emission-free production of hydrogen and carbon based fuels):
- Wu, Ji, Li, Yuan, Zhu, Wang*, Zhang, Licht, “Efficient, high yield carbon dioxide and water transformation to methane by electrolysis in molten salts,” Advanced Materials Technology, 1, 60092 (2016).
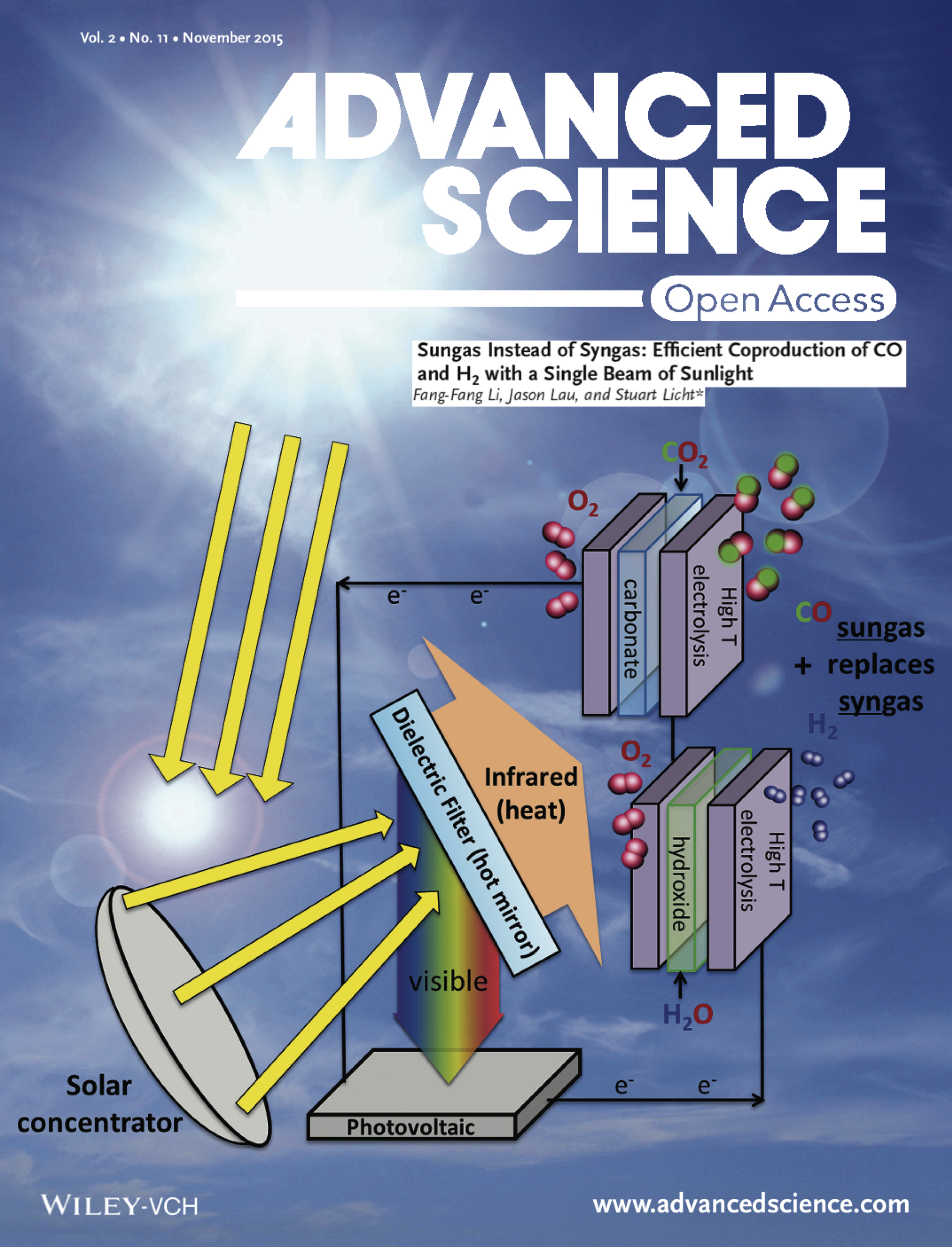
- Li, Lau, Licht " Sungas: instead of syngas: ... CO & H2 from a single beam of sunlight," Advanced Science, 2, 1500260 (2015). Click here to access article
- Licht, Liu, Cui, Lau, Hu, Stuart, Wang, El-Gazawi, Li, "Comparison of alternative molten electrolytes for water splitting to generate hydrogen fuel," J. Electrochem. Soc, 163, F1163 (2016). Click here to access article
- Li, Liu, Cui, Lau, Stuart, Licht, "A one-pot synthesis of H2 & carbon fuels from H2O & CO2" Advanced Energy Materials, 7, 140179 (2015).
- Licht, "Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach" Advanced Materials, 47, 5592-5612 (2011).
- Licht, "Solar thermal & efficient STEP H2 generation" in Solar Hydrogen & Nanotechnology, Wiley (2010).
- Licht, Chitayat, Bergmann, Dick, Ayub, Ghosh, "Efficient STEP (Solar Thermal Electrochemical Photo) Production of Hydrogen – an Economic Assessment," International J. Hydrogen Energy, 35, 10867 (2010).
STEP ammonia:
- Chen, Liu, Ha, Licht,* Gu.*, Li,* “Revealing nitrogen-containing species in commercial catalysts used for ammonia electrosynthesis” Nature Catalysis, 3, 1955 (2020); Click here for article
- Li, Licht, "Advances in understanding the mechanism and improved stability of the synthesis of ammonia from air and water...," Inorganic Chem., 53, 10042 (2014).
- Cui, Zhang, Liu, Liu, Xiang, Liu, Xin, Lefler Licht, "Electrochemical synthesis of ammonia directly from N2 and water over iron-based catalysts supported on activated carbons," Green Chemistry, 19, 298 (2017). Click here to access article
- Liu, Li, Wang, Peng, Licht, Licht, “Efficient Electrocatalytic Synthesis of Ammonia from Water and Air in a Membrane-Free Cell: Confining the Iron Oxide Catalyst to the Cathode,” European J. Inorg. Chem., DOI: 10.1002/ejic.201900667 (2019).
STEP iron (CO2 emission-free production of iron & carbon containing iron (steel)):
- Li, Wang, Licht, “Sustainable Electrochemical Synthesis of large grain or catalyst sized iron,” Journal of Sustainable Metallurgy, 2, 405 (2016). Click here to access article
- Licht, “STEP Iron: Producing Iron and Steel without CO2 Emissions” Ency. Iron & Steel, (in press 2015).
- Cui, Licht "Critical STEP advances for sustainable iron production," Green Chemistry, 15, 881 (2013).
- Licht, Wu, "STEP iron, a chemistry of iron formation without CO2 emission: Molten carbonate solubility and electrochemistry of iron ore impurities," J. Phys. Chem. C, 115, 25138 (2011).
- Licht, Wu, Zhang, Ayub, "Chemical Mechanism of the High Solubility Pathway for the Carbon Dioxide Free Production of Iron," Chemical Communications, 47, 3081 (2011).
- Licht, Wang, "High Solubility Pathway to the Carbon Dioxide Free Production of Iron," Chemical Communications, 46, 7004 (2010).
STEP Theory directs concentrated solar thermal energy to eliminate CO2 emission and decrease the energy of the electrolytic production of materials needed by society. STEP origins are from Licht theory & experiments on water splitting, using high temperature to tune the water splitting electrolysis potential to various semiconductor band-gap energies:
- Licht, "STEP: A solar chemical process to end anthropogenic global warming," J. Phys. Chem. C, 113, 16283-16292 (2009).
- Licht, "Efficient solar generation of hydrogen fuel - a fundamental analysis," Electrochem. Communications, 4/10, 789-794 (2002).
- Licht, "Solar water splitting to generate hydrogen fuel: photothermal electrochemical analysis," J. Phys. Chem. B, 107, 4253 (2003).
- Licht, Halperin, Kalina, Zidman, Halperin, "Electrochemical Potential Tuned Solar Water Splitting" Chemical Communications, 3006 (2003).
- Licht, "Thermochemical solar hydrgone generation" Chemical Communications, 4635 (2005).
- Licht, "Solar water splitting to generate hydrogen fuel - a photothermal electrochemical analysis," Int. J. Hydrogen Energy, 30, 459 (2005).
STEP cement (CO2 emission-free production of lime for cement):
- Licht, Wu, Hettige, Lau, Asercion, Stuart "STEP Cement: Solar Thermal Electrochemical Production of CaO without CO2 emission," Chemical Communications, 48, 6019 (2012).
- Licht, “Co-Production of Cement and Carbon Nanotubes with a Carbon Negative Footprint,” J. CO2 Utilization, 18, 378 (2017).
- Licht, Liu, Cui, Lau, Hu, Stuart, Wang, El-Gazawi, Li, "Comparison of alternative molten electrolytes for water splitting to generate hydrogen fuel," J. Electrochem. Soc, 163, F1163 (2016). Click here to access article
- Zhu, Wang, Wang, Liu, Wu, Licht, "The adoption and mechanism of KIO4 for redox-equilibrated stabilization of FeO42− as an equalizer in water," Ionics, 22, 1967 (2016). Click here to access article
- Wu, Ji, Li, Yuan, Zhu, Wang*, Zhang, Licht, “Efficient, high yield carbon dioxide and water transformation to methane by electrolysis in molten salts,” Advanced Materials Technology, 1, 60092 (2016).
Other STEP processes (magnesium, bleach, water treatment):
- Zhu, Wang, Wang, Liu, Wu, Licht, "Solar Thermoelectric Field Photocatlysis for Efficient Organic synthesis Exemplified by Toluene to Benzoic Acid," Applied Catalysis B, 193, 151-159 (2016).
- Licht, "Photoelectrochemical Conversion Processes" in Handbook of Electrochemistry, Springer (in press 2015).
- Zhu, Wang, Liu, Wang, Wu, Licht, "STEP Organic Synthesis," Green Chemistry, 16, 4758 (2014).
- Zhu, Wang, Liu, Wang Licht, "Towards efficient Solar STEP Synthesis Organic," Solar Energy (2015).
- Licht, “Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach” Advanced Materials, 47, 5592-5612 (2011).
- Licht, Wang, Wu, "STEP - A solar chemical process to end anthropogenic global warming II: experimental results," J. Phys. Chem., C, 115, 11803 (2011).
- Licht, "The solar thermal electrochemical production of energetic molecules: STEP" in Solar Energy Science & Eng. Appl., CRC (2013).
- Wang, Wu, Zhang, Licht, "STEP Wastewater Treatment," ChemSusChem, 5, 2000 (2012).
- Wang, Hu, W, Licht, " STEP Pollutant to Solar Hydrogen,” Electrochem. Sci. Lett., 2, H34 (2013).
 |
|
 |
 |
Photoelectrochemistry (light induced electrochemical charge transfer processes occuring at semiconductor electrolyte interfaces):
Gerischer probed fundamentals of "photoelectrochemical" illuminated semiconductor/electrolyte charge transfer in the mid-20th century, followed by photoelectrochemical water splitting in 1972 by Fujishima and Honda. Interest in the field intensified in the late 1970's with "wet" photoelectrochemical solar cells (PECs) by Hodes, Cahen, Manassen, Tenne, Wrighton, Heller, and Miller. The Licht group continued in this tradition as early pioneers in photoelectrochemistry including seminal studies in 1987 of a theory of solution chemistry controlled photoelectrochemical energetics (Cover article, Nature, 1987), and from 1987 onward high efficiency light invariant solar cells (Nature 1987), photoelectrochemical sensors (1996), efficient multiple bandgap) PECs in the 1990' , and highest efficiency PEC water splitting in the early 2000's. Licht's theory utilizing full spectrum sunlight, rather than semiconductor limited irradiation and solar thermal facilitated CO2-avoiding electrochemical syntheses of chemical staples led to his alternative STEP theory (Section III) and the group's efficient solar fuel program for generating H2, syngas and methane from air, water and sunlight (Section I) ongoing from 2002 to the present.
Licht group contributions to photoelectrochemistry
- Solution facilitated charge transfer for optimized photoelectrochemical energy conversion
Instead of solid-state photoelectrochemical charge transfer limitations, Licht's 1987 "A Description of Energy Conversion in Photoelectrochemical Solar Cells", introduced the importance of solution constrained charge transfer in PEC system. This approach isolates the rate determining reactive solution phase species, and led to demonstration of among the highest efficiency single crystal PEC solar cells (Nature 1990), and developments including Cs enhanced photovoltages and improved chalcogenides, used in contemporary solid and thin film solar cells.
- Light invariant solar cells "The solar cell that works in the dark!"
Solar energy is an abundant, but intermittent energy source. Photoelectrochemistry induces both electronic and electrochemical charge and the opportunity for combining in-situ electrochemical storage and solar conversion capabilities; providing continuous output insensitive to daily variations in illumination. A high solar to electric conversion efficiency cell configuration of this type was demonstrated first with Manassen, Hodes and Tenne in 1987 (Nature, 12%), with continued Licht group improvements reaching 18% solar conversion efficiency (Appl. Phys. Lett, 1999).
- Photoelectrochemical Sensors
The Licht group introduced a unique sensor for chemical species in liquids. The flatband potential of immersed, illuminated semiconductors varies with the activity interfacial species. Sweeping a constrained beam over the face of immersed semiconductors generates photovoltages which vary with the concentration at that distinct illuminated spatial region (Analytical Chem. 1996).
- Highest solar efficiency multiple bandgap photoelectrochemistry
Only a fraction of solar photons have sufficient energy for semiconductor excitation. To overcome this single bandgap limit, the Licht group developed a series of multiple bandgap PEC (MPEC) configurations in which adjacent bandgaps are bipolar or an inverted, have either ohmic or Schottky interface, and drive either electrical, chemical or electrochemical events (JPC feature article 2001).
- Solar Thermal Electrochemical Process (STEP)
As described in separate sections above, isolation of the semiconductor from the electrolytic interface, stabilizes the photoelectrochemical process, efficiency improvements by utilizing the full sunlight spectrum (including the previously wasted solar thermal spectrum) and permits high temperature “tuning & matching” of the solar/electrochemical energetics.
- Production of solar fuels (syngas, methane, and hydrogen from sunlight, air and water).
As described in a separate section above.
- Solar mitigation of the greenhouse gas carbon dioxide
As described in a separate section above.
Studies include:
- S. Licht, "A Description of Energy Conversion in Photoelectrochemical Solar Cells" Nature, 330, 148-151 (1987).
- S. Licht, G. Hodes, R. Tenne, J. Manassen, "A Light Variation Insensitive High Efficiency Solar Cell" Nature, 326, 863-864 (1987).
- S. Licht, D. Peramunage, "Efficient photoelectrochemical solar cells from electrolyte modification" Nature, 345, 330-333 (1990).
- S. Licht, D. Peramunage, "Efficiency in a liquid solar cell" Nature, 354, 440 (1991).
- S. Licht and V. Marcu, "A Potential and Charge Consistent Model for The Adsorption Dependence of Semiconductor Flat Band Potential" Journal of Electroanalytical and Interfacial Electrochemistry, 210, 197-204 (1986).
- S. Licht, Editor, 600 page monograph, Semiconductor Electrodes and Photoelectrochemistry,ISBN 3-527-30250-6 (Vol. 6 "Encyclopedia on Electrochemistry,") WILEY-VCH, Weinheim, Germany, (2002).
- S. Licht, "Multiple Bandgap Semiconductor/Electrolyte Solar Energy Conversion," Journal of Physical Chemistry, B, Feature Article, 105, 6281-6294 (2001).
- S. Licht, G. Hodes, Chapter 5, 36 pages: "Photoelectrochemical Storage Cells," in mongraph title: Photochemical and Photoelectrochemical Approaches to Solar Energy Conversion series title: Photoconversion of Solar Energy, Vol. 3, World Scientific Pub Co, London, ISBN: 978-1-86094-255-5,6 (2008).
- S. Licht, "Photoelectrochemistry," chapter Materials for Energy and Sustainability, D. Ginley and D. Cahen, editors, Cambridge University Press, Cambridge (2011).
- S. Licht,"Combined Solution Effects Yields Stable Thin Film Cd(Se,Te)/Polysulfide Photoelectrochemical Solar Cells" Journal of Physical Chemistry, 90, 1096-1099 (1986).
- S. Licht, F. Forouzan, "Solution Modified n-GaAs / Aqueous Polyselenide Photoelectrochemistry" Journal of the Electrochemical Society, 142, 1539-1545 (1995).
- S. Licht, N. Myung, "Aqueous Polyiodide Spectroscopy and Equilibria and its effect on n-WSe2 Photoelectrochemistry” Journal of the Electrochemical Society, 142, 845-849 (1995).
- S. Licht, N. Myung, Y. Sun, "A Light Addressable Photoelectrochemical Cyanide Sensor" Analytical Chemistry, 68, 954-959 (1996).
- S. Licht, D. Peramunage "Flat Band Variation of n-Cadmium Chalcogenides in Aqueous Cyanide" Journal of Physical Chemistry, 100, 9082-9087 (1996).
- S. Licht, O. Khaselev, P. A. Ramakrishnan, S. Faiman, E. A. Katz, A. Shames, S. Goren "Photoaction, Temperature and O2 depletion effects in Fullerene Photoelectrochemistry" Solar Energy Materials and Solar Cells, 56, 45-56 (1998).
- S. Licht, "Developments In Photoelectrochemistry: Light Addressable Photoelectrochemical Cyanide Sensors" Colloids and Surfaces A, 134, 231-239 (1998).
- S. Licht, B. Wang, T. Soga, M. Umeno "Light Invariant, Efficient, Multiple Bandgap AlGaAs/Si/Metal Hydride Solar Cell" Applied Physics Letters, 74, 4055-4057 (1999).
- B. Wang, S. Licht, T. Soga, M. Umeno "Stable Cycling Behavior of the Light Invariant AlGaAs/Si/Metal Hydride Solar Cell" Solar Energy Materials and Solar Cells, 64, 311-320 (2000).
- Licht, R. Tenne, H. Flaisher, J. Manassen, "A Pronounced Cation Effects on Performance and Stability of Cd-Chalcogenide Polysulfide Photoelectrochemical Cells" Journal of the Electrochemical Society, 131 950-951 (1984).
- Licht, "Comment on Adsorption of Hydroxide and Sulfide Ion on Single Crystal n-CdSe Electrodes" Journal of the Electrochemical Society, 132, 2801-2802 (1985).
- Levy-Clement, R. Triboulet, J. Rioux, S. Licht, R. Tenne, "Combined Solution Effects Yields Stable Increased Efficiency Thin Film Cd(Se,Te) Aqueous /Polysulfide Photoelectrochemical Solar Cells," Journal of the Electrochemical Society, 132, C361 (1985).
- Manassen, S. Licht, G. Hodes, D. Cahen, "A High Efficiency Thin Film Cadmium Chalcogenide/Polysulfide Photoelectrochemical Cell with In-Situ Storage" DOE Final Report, Solar Energy Research Institute, Golden Colorado (1985).
- Licht, J. Manassen, "The Effects of Hydroxide Ion on Cd-Chalcogenide/Polysulfide Photoelectrochemical Cells" Journal of the Electrochemical Society, 132, 1076-1081 (1985).
- Licht, R. Tenne, G. Dagan, J. Manassen, D. Cahen, R. Triboulet, J. Rioux, C. Levy-Clement, "High Efficiency n-Cd(Se,Te)/S=Photoelectrochemical Cell Resulting From Solution Chemistry Control" Applied Physics Letters, 46, 608-610 (1985).
- Licht, R. Tenne, G. Dagan, J. Manassen, D. Cahen, R. Triboulet, J. Rioux, C. Levy-Clement, "Control of Solution Chemistry Yields High Efficiency Stabilized n-Cd(Se,Te)/Polysulfide Photoelectrochemical Cell" Proc., Sixth European Photovoltaic Solar Energy Conference, London (1985).
- Levy-Clement, R. Triboulet, J. Rioux, S. Licht, R. Tenne, "Ternary n-Cd(Se,Te) Alloy Semiconductors: Synthesis, Material, Characterization and High Efficiency Photoelectrochemical Solar Cells," Journal of Applied Physics, 58, 4703-4708 (1985).
- Licht, J. Manassen, G. Hodes, "A Numerical Analysis of Aqueous Polysulfide Solutions" Inorganic Chemistry, 25, 2486-2489 (1986).
- Licht, "Cadmium Chalcogenide/Polysulfide Photoelectrochemical Cells with In-Situ Storage" Ph. D. Dissertation, The Weizmann Institute of Science, Rehovot, (1986).
- Licht, R. Tenne, H. Electrochemistry of Anions in Polysulfide Photoelectrochemical Cells" Journal of the Electrochemical Society, 133, 52-59 (1986).
- Licht, G. Hodes, J. Manassen, "The High Aqueous Solubility of Potassium Sulfide and Its Effect on Bulk and Photoelectrochemical Characterization of Cd(Se,Te)/Polysulfide Cells. I) Polysulfide Variation at Constant Sulfur/Sulfide Ratio" Journal of the Electrochemical Society, 133, 272-277 (1986).
- Licht, J. Manassen, "The High Aqueous Solubility of Potassium Sulfide and Its Effect on Bulk and Photoelectrochemical Characterization of Cd(Se,Te)/Polysulfide Cells. II) Variation of Sulfur/Sulfide Ratio" Journal of the Electrochemical Society, 133, 277-280 (1986).
- Manassen, D. Cahen, G. Hodes, R. Tenne, S. Licht, "The Importance of Solution Kinetics in Photoelectrochemical Phenomenon" Homogeneous and Heterogeneous Photocatalysis, Nato Series C, 174, 335-341 (1986).
- S. Licht, J. Manassen, "Thin Film Cadmium Chalcogenide/Aqueous Polysulfide Photoelectrochemical Solar Cells with In-Situ Storage" Journal of the Electrochemical Society, 134, 1064-1070 (1987).
- S. Licht, "Comment on Adsorption of Kinetics of Aqueous Polysulfide Solutions 1: Theory" Journal of the Electrochemical Society, 135, 258 (1988).
- S. Licht, "Comment on Adsorption of Kinetics of Aq. Polysulfide Solutions 2: Electrochem. Measurement" Journal of the Electrochemical Society, 135, 259 (1988).
- S. Licht, D. Peramunage, "High-Efficiency modified n-CdSe photoelectrochemical solar cell " Papers of the American Chemical Society, 199, 94 (1990).
- B. Miller, S. Licht, M. E. Orazem, P. C. Searson, "Photoelectrochemical Systems" 1991 Current Status of Surface Processing, NREL/CP-412-5007, USDOE, Chapt. 28, (1992).
- S. Licht, D. Peramunage, "Comment on Photoelectrochemistry" Journal of the Electrochemical Society, 139, 1792-1793 (1992).
- S. Licht, D. Peramunage, "Rational Electrolyte Modification of n-CdSe/(KFe(CN)63-/2- Photoelectrochemistry" Journal of the Electrochemical Society, 139, L23-L26 (1992).
- S. Licht, D. Peramunage, "Developments in n-Cdse/([Kfe(Cn)6]3-2-) Photoelectrochemistry” Papers of the American Chemical Society, 203, 315-part 2, (1992).
- S. Licht, D. Peramunage, "Photoelectrochemical Solar Energy Conversion an Emerging Renewable Energy Technology" ASME Solar Engineering, Vol. 2, 887-898 (1992).
- S. Licht, D. Peramunage, "Ligand, Cation and Speciation Effects on n-CdSe/([KFe(CN)6]3-/2-) Photoelectrochemistry" Solar Energy, 52, 197-204 (1994).
- B. Miller, S. Licht, M. E. Orazem, P. C. Searson, Photoelectrochemical systems" Critical Review Surface Chemistry, 3, 29-47 (1994).
- S. Licht, F. Forouzan, "Speciation Analysis of Aqueous Polyselenide Solutions" Journal of the Electrochemical Society, 142, 1546-1551 (1995).
- S. Licht, N. Myung, R. Tenne, G. Hodes, "Cation Electrolytic Modification of n-WSe2/Aqueous Polyiodide Photoelectrochemistry" Journal of the Electrochemical Society, 142, 840-844 (1995).
- S. Licht "Solution Aspects of Photoelectrochemistry" Solar Energy Materials and Solar Cells, 38, 353-354 (1995).
- S. Licht "Electrolyte Modified Photoelectrochemical Solar Cells" Solar Energy Materials and Solar Cells, 38, 305-319 (1995).
- N. Myung, S. Licht, "Potential Enhancement of Polyiodide Redox Couples via Solution Modification” Journal of the Electrochemical Society, 142, L129-L132 (1995).
- N. S. Lewis, A. J. Nozik, R. J. D. Miller, S.-F. Lindquist, J.-E. Moser, A. Hagfeldt, K. Uosaki, T. F. Schaafsma, S. Licht, H. Tributsch, F. Willig, "Comment on Photoelectrochemistry" Solar Energy Materials and Solar Cells, 38, 321-322 (1995).
- S. Licht, "Developments in photoelectrochemistry: Light addressable photoelectrochemical cyanide sensors” Papers of the American Chemical Society, 212, 149, (1996).
- S. Licht "Unusual Semiconductor Environmental Sensors for the Photoelectrochemical Detection of Cyanide" Environmental Aspects of Electrochemical Technology, M. Data et al., Editors, PV 96-21, Electrochemical Society, 113-125 (1996).
- S. Licht, "High Efficiency Solar Cells" Electrochemical Society Interface, 6, 34-39 (1997).
- S. Licht, J. Davis, "Disproportionation of Aqueous Sulfur and Sulfide: The Kinetics of Polysulfide Decomposition" Journal of Physical Chemistry, 101, 2540-2545 (1997).
- S. Licht, O. Khaselev, P. A. Ramakrishnan, S. Faiman, E. A. Katz, A. Shames, S. Goren, "Photoelectrochemical Investigation of Fullerenes" Fullerene Science and Technology, 6, (1998).
- S. Licht, O. Khaselev, P. A. Ramakrishnan, David Faiman, E. A. Katz, A. Shames, S. Goren, "Fullerene Photoelectrochemical Solar Cells" Solar Energy Materials and Solar Cells, 51, 9-19 (1998).
- S. Licht, B. Wang, "Efficiency Determination of Photoelectrochemical Solar Cells with In-Situ Storage," Reviews in Analytical Chemistry, 18, 301-310 (1999).
- S. Licht, O. Khaselev, T. Soga, M. Umeno, "Multiple Bandgap Photoelectrochemistry: Energetic Configurations For Solar Energy Conversion" Electrochemical and Solid State Letters, 1, 20-23 (1998).
- S. Licht, O. Khaselev, P. A. Ramakrishnan, T. Soga, M. Umeno, "Multiple Bandgap Photoelectrochemistry: Inverted Semiconductor Ohmic Regenerative Electrochemistry" Journal of Physical Chemistry,102, 2546-2554 (1998).
- S. Licht, O. Khaselev, P. A. Ramakrishnan, T. Soga, M. Umeno, "Multiple Bandgap Photoelectrochemistry: Bipolar Semiconductor Ohmic Regenerative Electrochemistry" Journal of Physical Chemistry, 102, 2536-2545 (1998).
- S. Licht, ChemInform: "Multiple Bandgap Semiconductor/Electrolyte Solar Energy Conversion," ChemInform, 32, issue 36-9-4-01 (2001).
- S. Licht, "Optimizing Photoelectrochemical Solar Energy Conversion: Multiple Bandgap and Solution Phase Phenomena" p. 358-393, in Semiconductor Electrodes and Photoelectrochemistry, Stuart Licht, Volume Editor, ISBN 3-527-30250-6 (Vol. 6 "Encyclopedia on Electrochemistry," A. Bard, Series Ed) WILEY-VCH, Weinheim, Germany, (2002).
- S. Licht, "Photoelectrochemical Solar Energy Storage Cells" p. 317-345, in Semiconductor Electrodes and Photoelectrochemistry, Stuart Licht, Editor, ISBN 3-527-30250-6 (Vol. 6 "Encyclopedia on Electrochemistry,") WILEY-VCH, Weinheim, Germany, (2002).
- Licht, “Efficient solar generation of hydrogen fuel – a fundamental analysis,” Electrochem. Communications, 4/10, 789-794 (2002).
- Licht, “Solar water splitting to generate hydrogen fuel: photothermal electrochemical analysis,” J. Phys. Chem. B, 107, 4253 (2003).
- Licht, Halperin, Kalina, Zidman, Halperin, “Electrochemical Potential Tuned Solar Water Splitting” Chemical Communications, 3006 (2003).
- Licht, “Thermochemical solar hydrogen generation” Chemical Communications, 4635 (2005).
- Licht, “Solar water splitting to generate hydrogen fuel – a photothermal electrochemical analysis,” Int. J. Hydrogen Energy, 30, 459 (2005).
- S. Licht "Energy Technology Division Research Award Address: Photoelectrochemical Storage of Solar Energy," Electrochemical Society Transactions, 2, 1-14 (2007).
- "The Solar Generation of Hydrogen: Towards a Renewable Energy Future," Monograph, 8 chapters, Editors: K. Rajeshwar, R. McConnell, S. Licht, Wiley Press, (2008).
- Rajeshwar, R. McConnnell, Harrionson, S. Licht, Chapt. 1, 18 pages: "ThevHydrogen Economy," in "The Solar Generation of Hydrogen: Towards a Renewable Energy Future,"Editors, Rajeshwar, McConnell, Licht, Wiley Press, (2008).
- Licht, Chapt. 5 "Thermochemical and Thermal/Photo Hybrid Solar Water Splitting," in "The Solar Generation of Hydrogen: Towards a Renewable Energy Future," Editors, Rajeshwar, McConnell, Licht, Wiley Press, (2008).
- S. Licht, "Phothermally and Thermally Assisted Photovoltaic," in Electrochemical Power Sources, 3, 350-368 (2009).
- Licht, "Solar thermal & solar thermal hybrid generation of hydrogen" Chapt. 21 in Monograph: Solar Hydrogen and Nanotechnology, editor: L. Vassieres, Wiley, 641-664 (2010).
- S. Licht, “Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach” Advanced Materials, 47, 5592-5612 (2011).
- Licht, "The Solar Thermal Electrochemical Production of energetic molecules: STEP," Chapt. 8 in Solar Energy Sciences and Engineering Applications, Eds.: Napoleon Enteria & Aliakbar Akbarzadeh, CRC Press, Published: December 10, 2013 (2013).
- S. Licht, "High solar efficiency utilization of CO2: The STEP (Solar Thermal Electrochemical Production) of energetic molecules Chapt. 6, p. 149-190, in Green Carbon Dioxide: Advances in CO2 Utilization, Ed.: Centi & Perathoner, Wiley, New Jersey (2014).
- Wu, Ji, Li, Yuan, Zhu, Wang*, Zhang, Licht, “Efficient, high yield carbon dioxide and water transformation to methane by electrolysis in molten salts,” Advanced Materials Technology, 1, 60092 (2016).
- Li, Lau, Licht, “Sungas: instead of syngas: …CO & H2 from a single beam of sunlight,” Advanced Science, 2, 1500260 (2015). Click here to access article
- Zhu, Wang, Wang, Liu, Wu, Licht, “Solar Thermoelectric Field Photocatlysis for Efficient Organic synthesis Exemplified by Toluene to Benzoic Acid,” Applied Catalysis B, 193, 151-159 (2016).
- S. Licht, "Photoelectrochemical Conversion Processes,” Chapter 24 in Springer Handbook of Electrochemistry, Eds. C Breitkopf, KaK.ren Swider Lyons, ISBN 3662466w, Springer New York (2017).
 |