J. Houston Miller, Greg B.Clarke, Hilary Melroy, Lesley Ott and Emily Wilson Steel
GeoCarbon:Towards an operational global carbon observing system; 24 Sep. 2013; Geneva; Switzerland
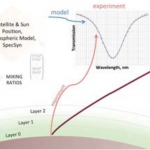
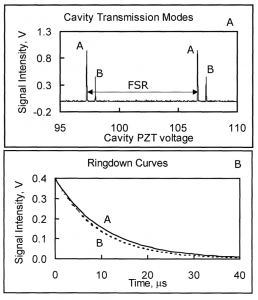
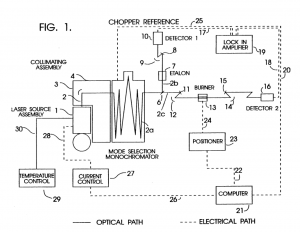
In a collaboration between NASA GSFC and GWU, a low-cost, surface instrument is being developed that can continuously monitor key carbon cycle gases in the atmospheric column: carbon dioxide (CO2) and methane (CH4). The instrument is based on a miniaturized, laser heterodyne radiometer (LHR) using near infrared (NIR) telecom lasers. Despite relatively weak absorption line strengths in this spectral region, spectrallyresolved atmospheric column absorptions for these two molecules fall in the range of 60-80% and thus sensitive and precise measurements of column concentrations are possible. In the last year, the instrument was deployed for field measurements at Park Falls, Wisconsin; Castle Airport near Atwater, California; and at the NOAA Mauna Loa Observatory in Hawaii. For each subsequent campaign, improvement in the figures of merit for the instrument has been observed. In the latest work the absorbance noise is approaching 0.002 optical density (OD) noise on a 1.8 OD signal. An overview of the measurement campaigns and the data retrieval algorithm for the calculation of column concentrations will be presented. For light transmission through the atmosphere, it is necessary to account for variation of pressure, temperature, composition, and refractive index through the atmosphere that are all functions of latitude, longitude, time of day, altitude, etc. For temperature, pressure, and humidity profiles with altitude we use the Modern-Era Retrospective Analysis for Research and Applications (MERRA) data. Spectral simulation is accomplished by integrating short-path segments along the trajectory using the SpecSyn spectral simulation suite developed at GW. Column concentrations are extracted by minimizing residuals between observed and modeled spectrum using the Nelder-Mead simplex algorithm. We will also present an assessment of uncertainty in the reported concentrations from assumptions made in the meteorological data, LHR instrument and tracker noise, and radio frequency bandwidth and describe additional future goals in instrument development and deployment target