Hannah Caplan
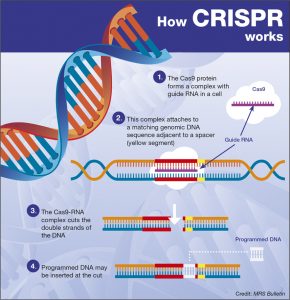
Genome editing refers to a group of technologies that allow scientists to edit DNA sequences.1 Clustered regularly interspaced short palindromic repeats, CRISPR, is the newest genome editing technology. CRISPR provides scientists with a faster, less expensive, and more accurate means of modifying DNA than ever before.1 Genome editing in healthcare can offer patients new cures and potential cost-savings by treating the genetic source of a disease. CRISPR associated proteins, often CRISPR associated protein 9 (CRISPR-Cas9), interact with guide RNA (gRNA) to identify the segment of DNA that contains the mutated gene.2 Scientists can reprogram the guide RNA to identify a new target gene, making this technique simpler to execute than previous gene editing techniques, such as ZNF and TALENS.3 Once the guide RNA and Cas9 identify the segment of DNA, Cas9 binds to and slices both strands of DNA, effectively shutting off the expression of that gene.2 Scientists can then perform insertions and deletions at the site of the break to make changes in the DNA. More recent research has also demonstrated that CRISPR-Cas9 can be used to change DNA base pairs without cutting the DNA, reducing the possibility of technical errors.4
Scientists at Stanford University recently demonstrated the effectiveness of CRISPR technology to target and repair the genetic mutation responsible for sickle cell anemia.5 The scientists successfully modified the gene in blood stem cells, known as hematopoietic cells, that causes red blood cells to produce sickle shaped hemoglobin.5 Once the scientists replaced the mutated segment with the correct DNA sequence, the red blood cells produced normal-shaped hemoglobin.5 After the body produces enough normal-shaped hemoglobin, the red blood cells can properly circulate oxygen throughout the body, thereby curing the patient of sickle cell anemia.6
Genome editing has become a prominent topic among policy makers considering the potential long-term effects and ethical concerns associated with gene editing. While many scientists have so far demonstrated successful applications of genome editing techniques, little information is available on the long-term effects of genome interference. In addition, scientists could wrongly identify the point of mutation along a strand of DNA and in doing so, might alter an otherwise healthy DNA sequence. A variety of ethical concerns also arise when discussing the acceptability of genome editing. Genome editing techniques can be used to alter DNA in somatic cells also known as the body cells. However, genome editing techniques could also be used to alter other cells. Parents may be interested in genetically modifying their egg or sperm cells to prevent passing down an inheritable condition to their child. However, ethicists must consider the extent to which it is acceptable to genetically modify the human genome. Many of these concerns fall under the authority of the Food and Drug Administration (FDA).7 The FDA regulates gene editing in somatic cells under their existing framework for biologic products.7 Genetic modification of human embryos falls under a separate category and is subject to much stricter regulations.7 With this new technology we have the potential to create a healthier generation, but this also brings up concerns for designer babies and the limits to gene editing. Furthermore, the cost of genome editing technology and access to care issues are still unknown. This new technology could further exacerbate the health disparities already seen between socioeconomic classes in the United States.
References
- What are genome editing and CRISPR-Cas9? National Institutes of Health: Genetics Home Reference. Available at: https://ghr.nlm.nih.gov/primer/genomicresearch/genomeediting
- Questions and answers about CRISPR. Broad Institute. Available at: https://www.broadinstitute.org/what-broad/areas-focus/project-spotlight/questions-and-answers-about-crispr
- Gupta, R. M., & Musunuru, K. (2014). Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. The Journal of clinical investigation, 124(10), 4154.
- New CRISPR gene editors can fix RNA and DNA one typo at a time. Science News. Available at: https://www.sciencenews.org/article/new-crispr-gene-editors-can-fix-rna-and-dna-one-typo-time
- Researchers take step toward gene therapy for sickle cell disease. Stanford Medicine. Available at: https://med.stanford.edu/news/all-news/2016/11/researchers-take-step-toward-gene-therapy-for-sickle-cell-disease.html
- What is sickle cell disease? National Institutes of Health. Available at: https://www.nhlbi.nih.gov/health/health-topics/topics/sca
- Califf, R. M., & Nalubola, R. (2017). FDA’s Science-Based Approach to Genome-Edited Products. US Food and Drug Administration. Available at: https://blogs.fda.gov/fdavoice/index.php/2017/01/fdas-science-based-approach-to-genome-edited-products/
- Ball, Philip. (2016). CRISPR: Implications for materials science [Digital image]. Retrieved from https://www.cambridge.org/core/journals/mrs-bulletin/news/crispr-implications-for-materials-science
 Hannah is a senior majoring in Public Health at the George Washington University Milken Institute School of Public Health.
Hannah is a senior majoring in Public Health at the George Washington University Milken Institute School of Public Health.