We aim to develop catalytic processes that contribute to a circular economy, i.e. convert renewables into benign, functional and degradable chemicals. This necessitates concerted efforts to both develop new catalytic processes and design functional chemicals that are inherently benign and either non-persistent, or degradable chemically, as illustrated below:
 Exploring both process development and chemical design allows us to capitalize on the strong nexus between the two fields, and to arm young chemists with a more comprehensive skill set for developing sustainable technologies. Our group’s research contributes to the two research frontiers by 1) developing new defunctionalization processes facilitated by active, selective and robust supported catalysts and 2) developing tools for the prediction of human and ecological toxicity that help enable the design of safer chemicals (follow link).
Exploring both process development and chemical design allows us to capitalize on the strong nexus between the two fields, and to arm young chemists with a more comprehensive skill set for developing sustainable technologies. Our group’s research contributes to the two research frontiers by 1) developing new defunctionalization processes facilitated by active, selective and robust supported catalysts and 2) developing tools for the prediction of human and ecological toxicity that help enable the design of safer chemicals (follow link).
Multifunctional catalysts on non-innocent supports
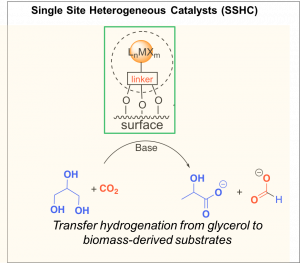
We are currently exploring novel multifunctional catalysts that can facilitate challenging synthetic and energy-related transformations by taking advantage of proximity effects of site-isolated organometallic species to acid/base active sites on an appropriate support. If the electronic properties of the support can be finely tuned, this strategy also allows for tuning of the electronic properties and reactivity of the organometallic species via “macroligand” support effects. This dual strategy affords the opportunity to significantly change both the reactivity and lifetime of site-isolated species, thus overcoming the traditional challenges of supported catalysts – decreased activity and lifetime compared to homogeneous counterparts.
Two classes of supports that allow electronic tuneability are mixed metal oxides (MMOs) and their precursors, layered double hydroxides (LDHs). The two supports offer distinct strength of binding, accessibility of the organometallic species and electronic effect of the support. Our early work investigated the surface chemistry of LDHs and their suitability for serving as supports for organic moieties (Finn et al, 2015).
Transfer Hydrogenation for Biomass Valorization
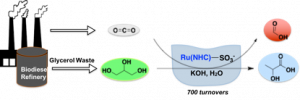
Most recently, we have developed an application for this type of supported catalysts – the transfer hydrogenation of CO2 and from glycerol, affording formic and lactic acid (Heltzel et al, 2018). Carbonate salts can also be utilized in place of CO2, affording the same products at higher rates. This process is a highly attractive path to valorizing two waste streams and is a significantly more thermodynamically favorable process than direct CO2 hydrogenation. We reported the first homogenoues catalyst for this process, and are currently designing more active homogeneous species and investigating the mechanism. We shall shall shortly report on the supported catalysts for this process, composed of immobilized site-isolated Ru and Ir species on doped layered double hydroxide supports.
Acceptorless Dehydrogenation Strategies for Biomass Valorization
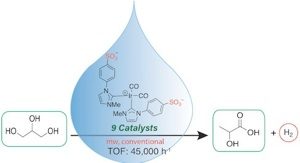
To this end, we have also recently shown that the catalysts being explored for CO2 transfer hydrogenation from glycerol are highly active for glycerol dehydrogenation under basic conditions, to afford lactic acid with very high turnover numbers. (Finn et al, 2018). By overcoming the solubility challenge associated with known homogeneous catalysts for this reaction, we show that thermally robust Ir(I), Ir(III), and Ru(II) N-heterocyclic carbene (NHC) complexes with sulfonate-functionalized wingtips are highly prolific for this process, requiring no co-solvents other than aqueous base. The most active catalyst reaches a TOF of 45 592 h–1 (microwave) and 3477 h–1 (conventional heating). The most active catalyst retains equal activity for crude glycerol. A mechanism is proposed for the most active catalyst precursor involving O–H oxidative addition of glycerol.
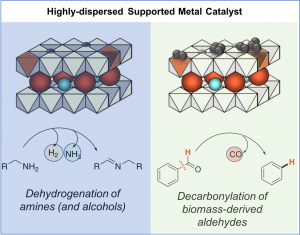
 In addition to single-site catalysts, we are also exploring highly dispersed clusters and nanoparticles immobilized in tunable HT matrices. For example, we have developed a series of Pd-doped layered double hydroxides (Pd-HTs), which consist of Bronsted basic and Lewis acidic surface sites that surround Pd species in 0, 2+ and 4+ oxidation states. Modulating Pd loading allows adjustment of Pd speciation and optimization of dehydrogenation activity. Applications of these catalysts include acceptorless amine dehydrogenation, which affords imines from secondary amines, and secondary amines from primary amines with low catalyst loading (0.5 mol%) (Ainembabazi et al, 2019). Poisoning data suggests Pd-HTs are primarily operationally heterogeneous. Leaching is negligible, and catalyst can be regenerated by acid dissolution and re-precipitation. These catalysts are also highly active for decarbonyaltion of aldehydes, especially ones derived from biomass, such as 5-hydroxymethylfufural (HMF) and furfural (An et al, ChemSusChem 2019).
In addition to single-site catalysts, we are also exploring highly dispersed clusters and nanoparticles immobilized in tunable HT matrices. For example, we have developed a series of Pd-doped layered double hydroxides (Pd-HTs), which consist of Bronsted basic and Lewis acidic surface sites that surround Pd species in 0, 2+ and 4+ oxidation states. Modulating Pd loading allows adjustment of Pd speciation and optimization of dehydrogenation activity. Applications of these catalysts include acceptorless amine dehydrogenation, which affords imines from secondary amines, and secondary amines from primary amines with low catalyst loading (0.5 mol%) (Ainembabazi et al, 2019). Poisoning data suggests Pd-HTs are primarily operationally heterogeneous. Leaching is negligible, and catalyst can be regenerated by acid dissolution and re-precipitation. These catalysts are also highly active for decarbonyaltion of aldehydes, especially ones derived from biomass, such as 5-hydroxymethylfufural (HMF) and furfural (An et al, ChemSusChem 2019).
For more recent publications see Publications.