August 20, 2017
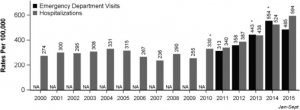
Nearly 2.7 million patients present to the emergency department (ED) annually with acute low back pain (LBP). Current medical practice dictates that specific nonsteroidal anti-inflammatory drugs (NSAIDs) are an efficacious first-line therapy. However, uncertainty remains on whether the addition of other classes of medication (i.e., combination therapy) can further improve LBP outcomes. Currently, benzodiazepines (e.g., diazepam) are considered a prescriber favorite to accompany NSAID therapy and are prescribed in 300,000 US ED visits for LBP, annually.[i]
How We Got Here
In 2014, Williams et al. published a practice-changing study in The Lancet showing that neither regular, nor as-needed acetaminophen dosing affects recovery time when compared with placebo in LBP.[ii] Since then, naproxen has become a standard over-the-counter (OTC) medication for acute LBP. Friedman et al. (2014) added to the evidence surrounding care of acute LBP by showing that under the same presentation, adding cyclobenzaprine or oxycodone/acetaminophen to naproxen therapy does not improve functional outcomes.[iii] A 2017 study published in Annals of Emergency Medicine by Friedman et al. raises the question:
Will adding a short-term course of diazepam to naproxen yield any additional benefit to functional outcomes at one-week post-discharge from the ED in patients with acute LBP?[iv]
An Urgent Matters podcast with Dr. Friedman details the study as well as how it may or may not change emergency medicine practice.[v]
Friedman et al. 2017
Dr. Friedman and his team conducted a randomized, double-blind, comparative efficacy clinical trial for adult patients presenting to the ED with acute (onset <2 weeks), atraumatic, non-radicular LBP and functional impairment quantified by a Roland-Morris Disability Questionnaire (RMDQ) greater than 5. The RMDQ is a validated health status measure for LBP in both research and clinical practice consisting of 24 Yes/No statements, each corresponding as one point if marked “Yes,” and no point if marked “No.” Both intervention and control group received a 10-minute LBP educational session and naproxen 500mg PO BID as-needed. The intervention group received either 28 tablets of diazepam 5mg or identical placebo to be taken BID as-needed.
- Intervention Group: Education + 500mg Naproxen BID (as needed) + 5mg Diazepam BID (as-needed)
- Control Group: Education + 500mg Naproxen BID (as needed) + Placebo BID (as-needed)
While many outcomes were measured starting at the point of discharge, the primary outcome measured was a change in RMDQ score between ED discharge and one-week follow-up. A decrease of 5 or more points when compared to the other group was considered clinically significant. The secondary outcome measures included pain intensity on a subjective four-point descriptive scale (none, mild, moderate, and severe) at one-week and three-month checkpoints and medication adherence for LBP within 72 hours of ED discharge. Only 21% (114/545) of the patients screened were included in the study after meeting selection criteria. The mean RMDQ score of both patients randomized to the intervention and control group improved by 11 points. The study found no comparative improvement in functional outcome at one week when diazepam was added to a naproxen regimen. Additionally, pain intensity at one week and three months was no different between the two groups, with similar incidence of adverse events throughout the trial.
While limitations to the study certainly exist, results appear reasonable when taken in context of the reality behind clinical practice. Most noteworthy of the limitations is that the study was conducted in a single urban healthcare system serving a population with predominantly low socioeconomic status (SES). Lower neighborhood median household incomes and higher unemployment rates have been associated with longer length of disability (i.e., longer prognosis), potentially impacting outcome data.[vi] Secondly, in the interest of maintaining cohort homogeneity, researchers excluded many patients secondary to strict entry criteria. Therefore, there is a reasonable index of suspicion about the study’s generalizability to patients with other types of back pain. Anecdotally speaking, often times the onset of radiating pain can serve as the impetus behind seeking emergency care. Finally, it is important to note that while diazepam is also prescribed as an anxiolytic, this would have skewed data in favor of the intervention group in controlling LBP (not the case here). In the future, research should be done on the efficacy of alternative treatments for LBP such as controlled stretching and thermal pack use.
Key Takeaways
Diazepam does not have marginal effectiveness when prescribed alongside naproxen for outpatient management of atraumatic, nonradicular LBP following ED discharge when it comes to functional outcomes at one week and pain outcomes at one and three months. Patients should routinely be counseled on the potential for benign symptoms to persist, encouraged to use the recommended dose of naproxen as-needed, and return to the ED with any significant changes.
“Physicians should reassure their patients that acute and subacute low back pain usually improves over time regardless of treatment.” - ACP President Nitin Damle, MD MS
Barriers to Implementation
The often benign symptoms of acute atraumatic, nonradicular LBP and the subjective nature of reporting pain lead to frustration for both patient and provider. Additionally, clinically irrelevant but psychologically important factors such as patients expectation for prescriptions after physician interaction are detrimental to prescriber stewardship.[vii] Physicians may also feel pressure to order additional testing – for this, the Choosing Wisely initiative is a great resource to aid physicians in identifying patients that warrant further testing.
[i] Friedman BW, Chilstrom M, Bijur PE, Gallagher EJ. Diagnostic testing and treatment of low back pain in US emergency departments. A national perspective. Spine. 2010;35(24):E1406-E1411. doi:10.1097/BRS.0b013e3181d952a5.
[ii] Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial Williams, Christopher M et al. The Lancet , Volume 384 , Issue 9954 , 1586 - 1596
[iii] Friedman BW, Dym AA, Davitt M, Holden L, Solorzano C, Esses D, Bijur PE, Gallagher EJ. Naproxen With Cyclobenzaprine, Oxycodone/Acetaminophen, or Placebo for Treating Acute Low Back PainA Randomized Clinical Trial. JAMA. 2015;314(15):1572–1580. doi:10.1001/jama.2015.13043
[iv] Diazepam Is No Better Than Placebo When Added to Naproxen for Acute Low Back Pain Friedman, Benjamin W. et al. Annals of Emergency Medicine , Volume 70 , Issue 2 , 169 - 176.e1
[v] Pines JM. – Acute Low Back Pain: Diazepam No Better Than Placebo? with Dr. Benjamin Friedman. Urgent Matters Podcast. 2017
[vi] Shraim M, Cifuentes M, Willetts JL, Marucci‐Wellman HR, Pransky G. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. American Journal of Industrial Medicine. 2017;60(5):472-483. doi:10.1002/ajim.22712.
[vii] Sirota, M., Round, T., Samaranayaka, S., & Kostopoulou, O. Expectations for antibiotics increase their prescribing: Causal evidence about localized impact. Health Psychology, 36(4), 402-409.
Ameer Khalek is a MPH student at the GWU Milken Institute School of Public Health